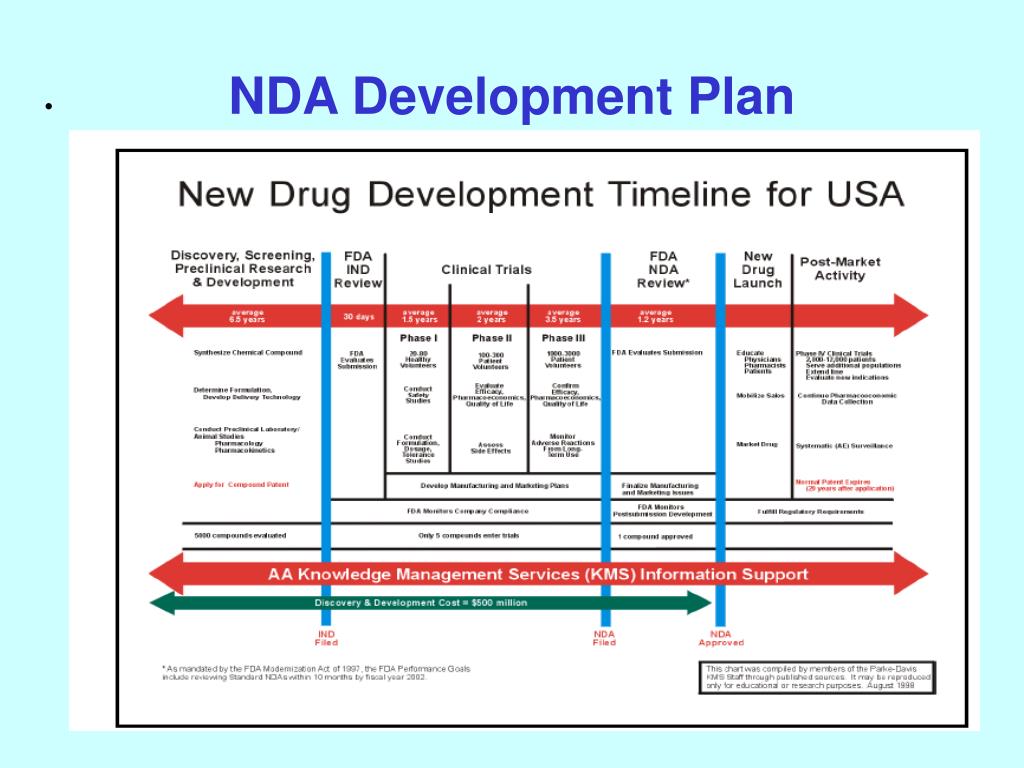
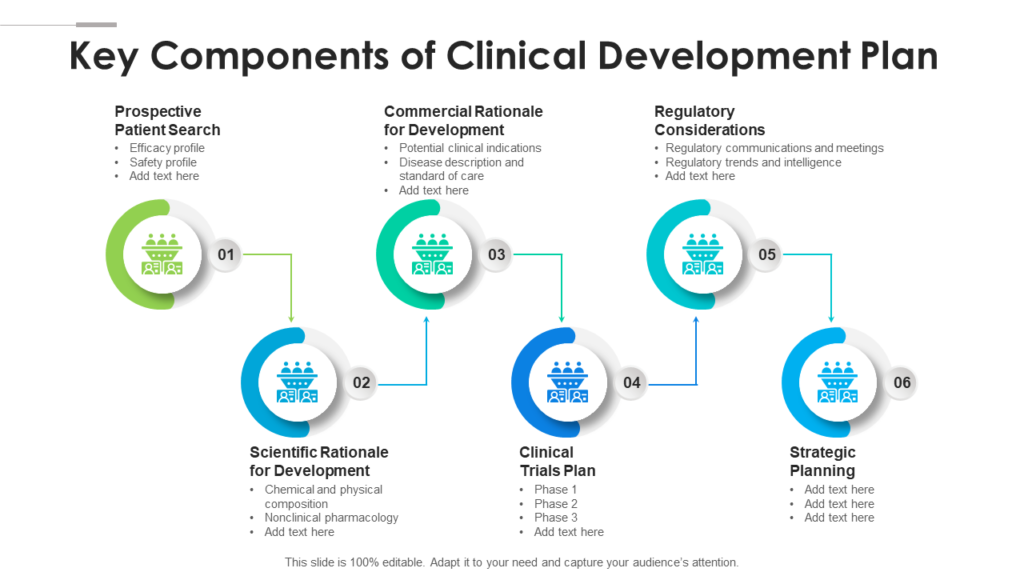
Clinical Development Plan Template Fda
Clinical Development Plan Template Fda - Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism Ind content and format for phase 1 studies. Guidance for industry content and format of investigational new drug applications (inds). A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Ensure medical device success with strategic planning, risk management, and compliance. The clinical development plan guide there are two essential documents that any company developing a new pharmaceutical or.
Ensure medical device success with strategic planning, risk management, and compliance. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism Ind content and format for phase 1 studies. Guidance for industry content and format of investigational new drug applications (inds). A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. The clinical development plan guide there are two essential documents that any company developing a new pharmaceutical or.
Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism Guidance for industry content and format of investigational new drug applications (inds). Ind content and format for phase 1 studies. Ensure medical device success with strategic planning, risk management, and compliance. A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. The clinical development plan guide there are two essential documents that any company developing a new pharmaceutical or.
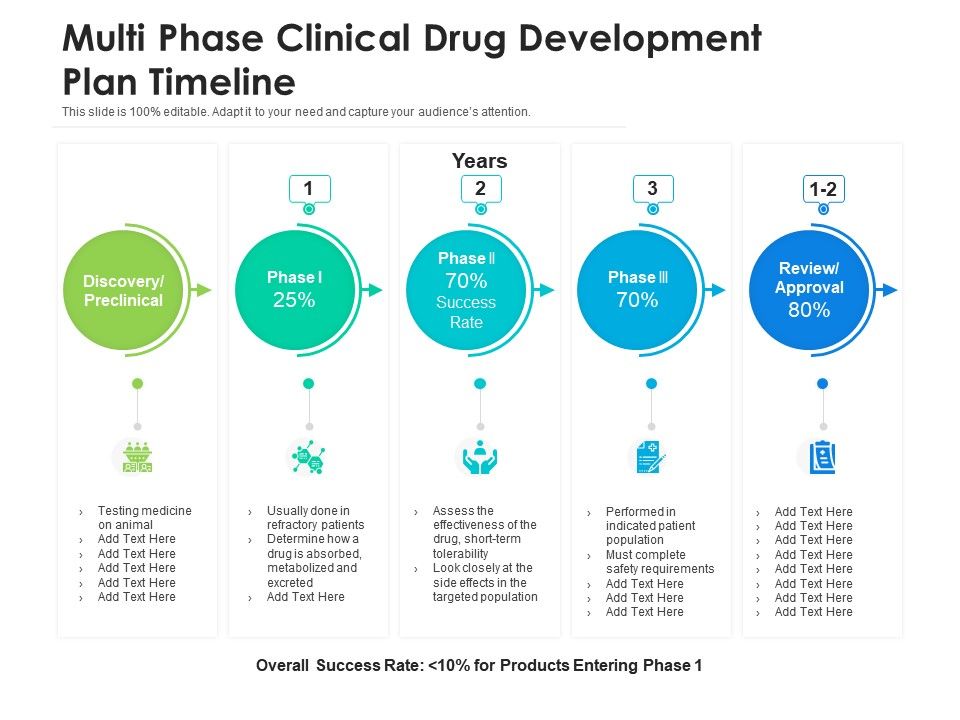
Clinical Development Plan Template Venngage
Guidance for industry content and format of investigational new drug applications (inds). Ensure medical device success with strategic planning, risk management, and compliance. Ind content and format for phase 1 studies. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug.
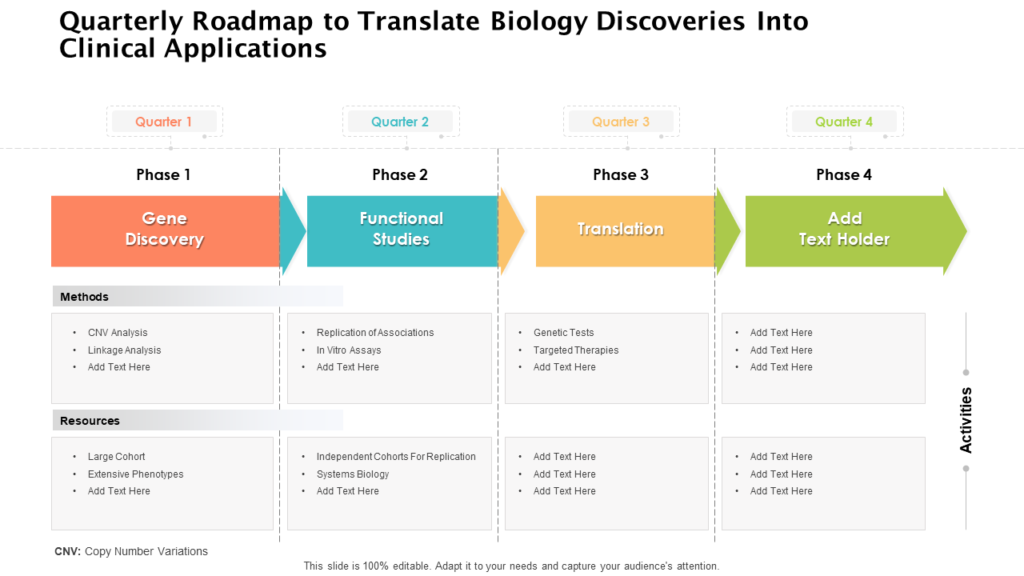
Clinical Development Plan Template Fda
Ensure medical device success with strategic planning, risk management, and compliance. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism The clinical development plan guide there are two essential documents that any company developing a new pharmaceutical or. Guidance for industry content and format of investigational new drug applications (inds). A target product profile (tpp) is a.
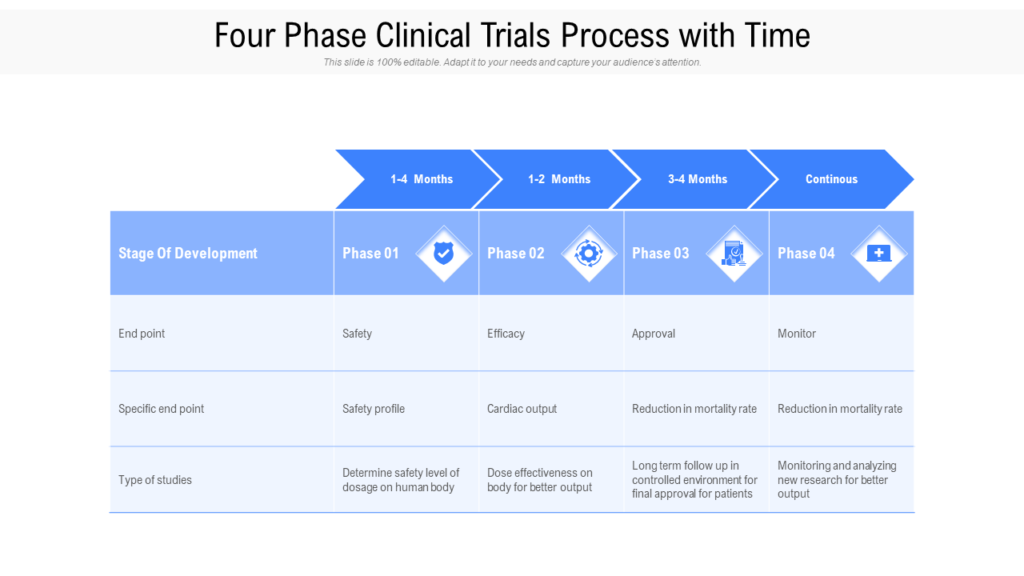
Clinical Development Plan Template
The clinical development plan guide there are two essential documents that any company developing a new pharmaceutical or. Guidance for industry content and format of investigational new drug applications (inds). Ensure medical device success with strategic planning, risk management, and compliance. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism A target product profile (tpp) is a.
Clinical Development Plan Template Fda
The clinical development plan guide there are two essential documents that any company developing a new pharmaceutical or. Ind content and format for phase 1 studies. Ensure medical device success with strategic planning, risk management, and compliance. A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Clinical investigational plan synopsis.
Clinical Development Plan Template in Google Docs, Pages, Word
Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism Ind content and format for phase 1 studies. The clinical development plan guide there are two essential documents that any company developing a new pharmaceutical or. Ensure medical device success with strategic planning, risk management, and compliance. Guidance for industry content and format of investigational new drug applications.
Clinical Development Plan Template
Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Guidance for industry content and format of investigational new drug applications (inds). The clinical development plan guide there are two essential documents that any company developing a new pharmaceutical.
Clinical Development Plan Template Fda prntbl.concejomunicipaldechinu
The clinical development plan guide there are two essential documents that any company developing a new pharmaceutical or. A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Ind content and format for phase 1 studies. Guidance for industry content and format of investigational new drug applications (inds). Ensure medical device.
Clinical Development Plan Template Fda prntbl.concejomunicipaldechinu
Guidance for industry content and format of investigational new drug applications (inds). A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Ind content and format for phase 1 studies. Ensure medical device success with strategic planning, risk management, and compliance. The clinical development plan guide there are two essential documents.
Top 20 PowerPoint Templates to Create a Clinical Development Plan
Ensure medical device success with strategic planning, risk management, and compliance. A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism Ind content and format for phase 1 studies. Guidance for industry content and format of investigational new drug.
Clinical Development Plan Template Fda
Guidance for industry content and format of investigational new drug applications (inds). Ensure medical device success with strategic planning, risk management, and compliance. The clinical development plan guide there are two essential documents that any company developing a new pharmaceutical or. A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process..
Clinical Investigational Plan Synopsis • New Onset Atrial Fibrillation • Pulmonary Embolism
A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Guidance for industry content and format of investigational new drug applications (inds). Ind content and format for phase 1 studies. The clinical development plan guide there are two essential documents that any company developing a new pharmaceutical or.