Isotopes Practice Worksheet
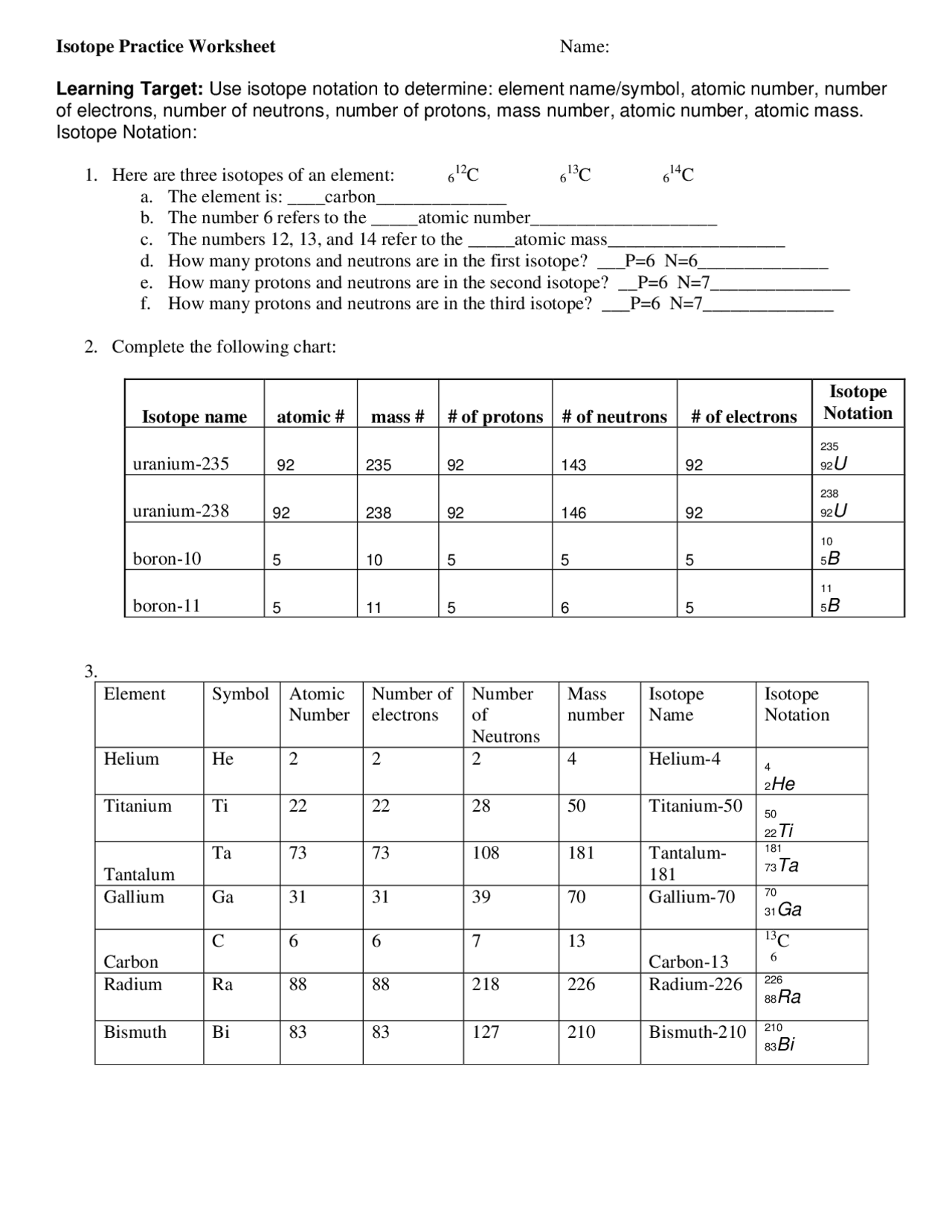
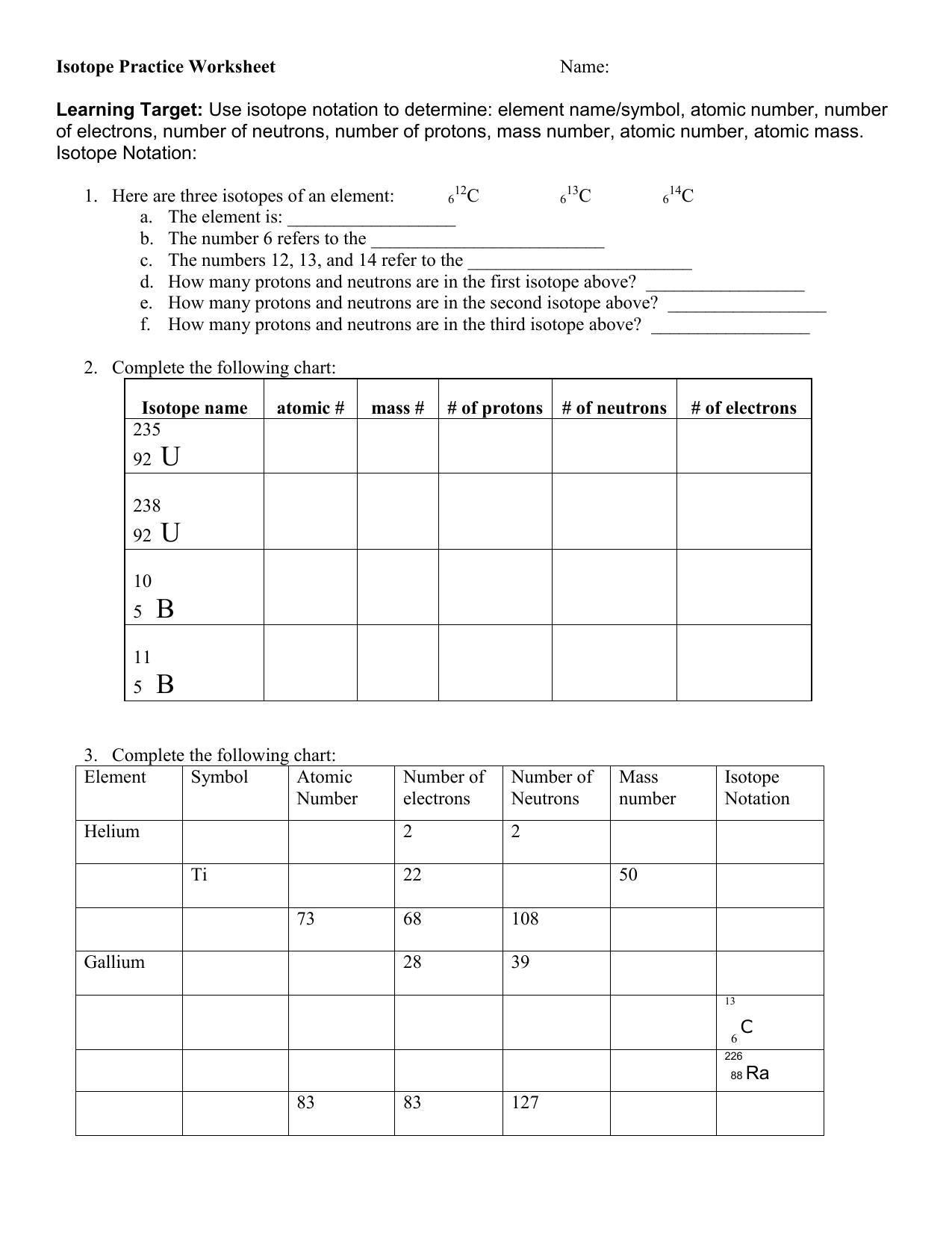
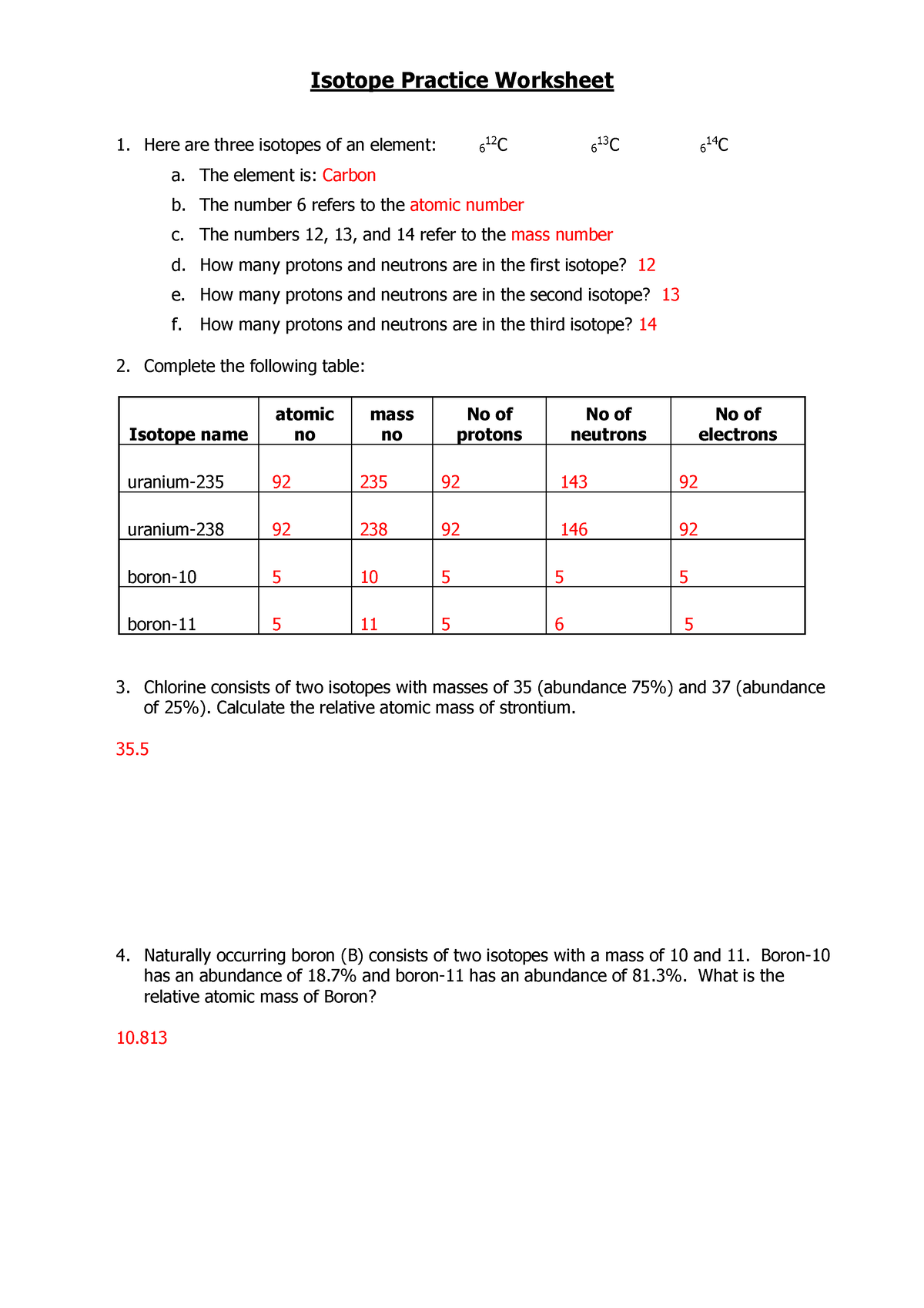
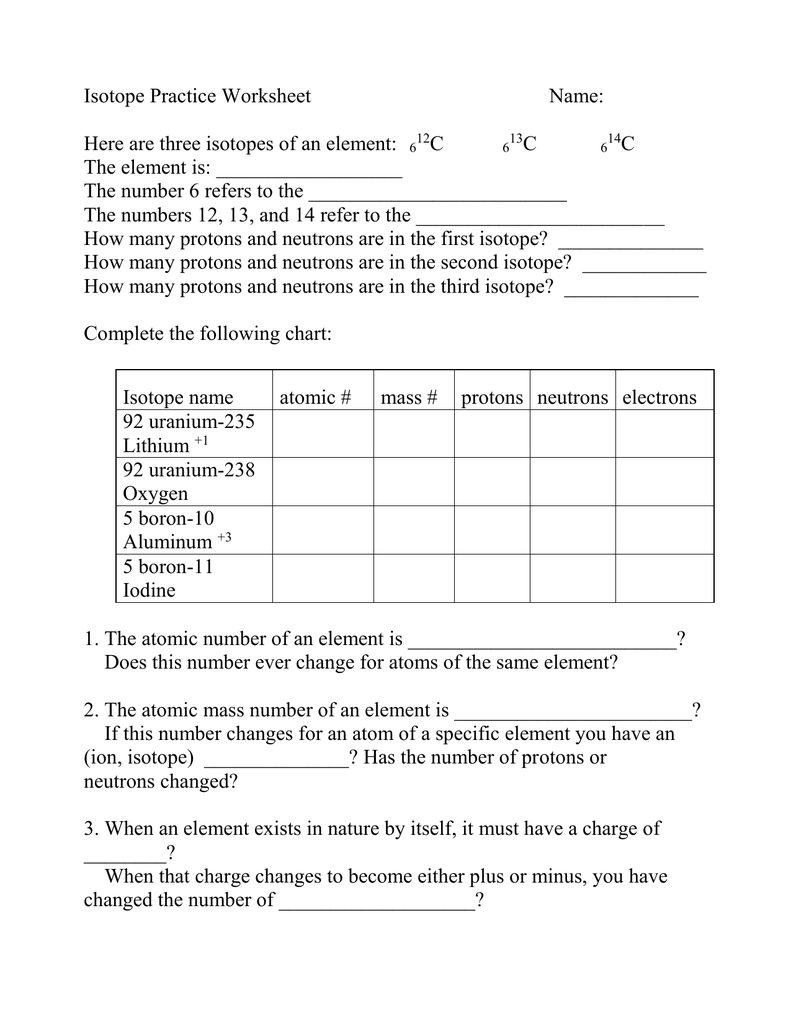
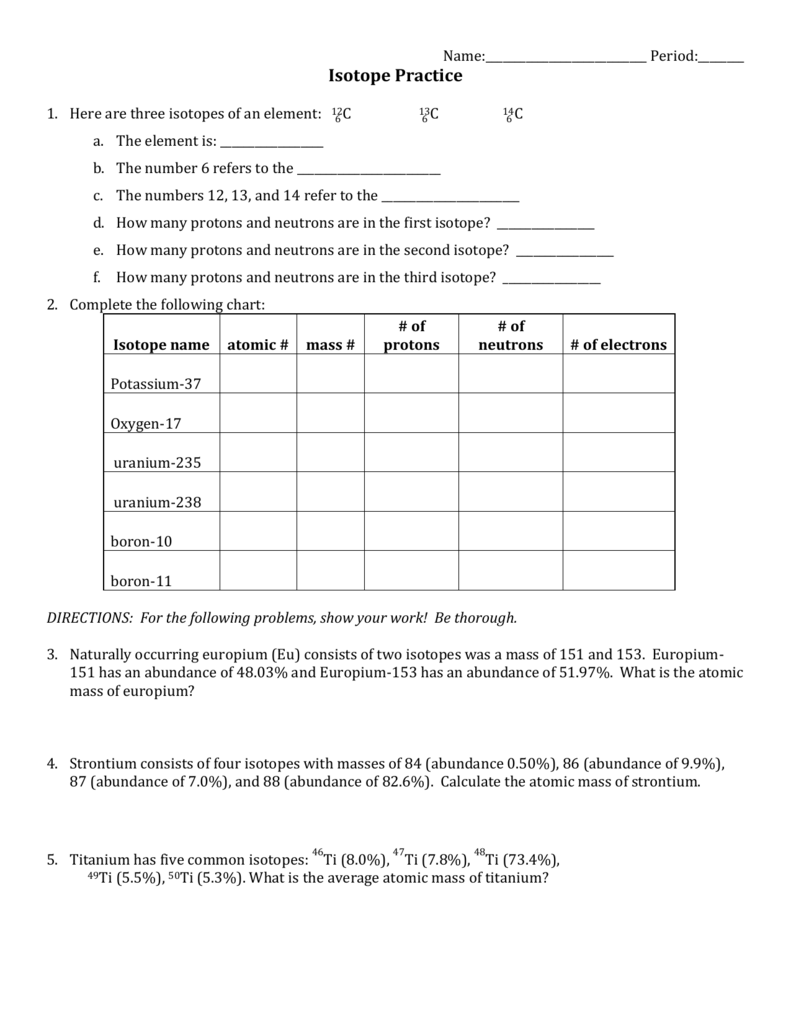
Isotopes Practice Worksheet - The average atomic mass of a lead atom is 207.2 amu. Complete the following table, using the periodic table in the back of your book. What does the number next to isotopes signify? Students will practice how to write isotopes and how to calculate the number of subatomic particles based on atomic mass. Which isotope of lead is. For each of the following isotopes, write the # of protons, neutrons, and electrons. Here are three isotopes of an element: The number 6 refers to the _____ c. Fill in the isotope names and any missing information, including.
Complete the following table, using the periodic table in the back of your book. For each of the following isotopes, write the # of protons, neutrons, and electrons. Here are three isotopes of an element: Which isotope of lead is. Fill in the isotope names and any missing information, including. The average atomic mass of a lead atom is 207.2 amu. Students will practice how to write isotopes and how to calculate the number of subatomic particles based on atomic mass. The number 6 refers to the _____ c. What does the number next to isotopes signify?
Here are three isotopes of an element: Students will practice how to write isotopes and how to calculate the number of subatomic particles based on atomic mass. Which isotope of lead is. The number 6 refers to the _____ c. For each of the following isotopes, write the # of protons, neutrons, and electrons. Fill in the isotope names and any missing information, including. Complete the following table, using the periodic table in the back of your book. The average atomic mass of a lead atom is 207.2 amu. What does the number next to isotopes signify?
Isotope Practice Worksheet Lecture notes Chemistry Docsity
The average atomic mass of a lead atom is 207.2 amu. Fill in the isotope names and any missing information, including. What does the number next to isotopes signify? The number 6 refers to the _____ c. Students will practice how to write isotopes and how to calculate the number of subatomic particles based on atomic mass.
Practice Isotope Calculations 2 Answer Key Practice Isotope
For each of the following isotopes, write the # of protons, neutrons, and electrons. Which isotope of lead is. Students will practice how to write isotopes and how to calculate the number of subatomic particles based on atomic mass. Fill in the isotope names and any missing information, including. Complete the following table, using the periodic table in the back.
Chemistry Worksheet Isotope Notation Chemistry Worksheet Iso
Fill in the isotope names and any missing information, including. Here are three isotopes of an element: The number 6 refers to the _____ c. Students will practice how to write isotopes and how to calculate the number of subatomic particles based on atomic mass. For each of the following isotopes, write the # of protons, neutrons, and electrons.
Isotopes And Nuclear Chemistry Worksheet
For each of the following isotopes, write the # of protons, neutrons, and electrons. Which isotope of lead is. Here are three isotopes of an element: What does the number next to isotopes signify? The average atomic mass of a lead atom is 207.2 amu.
Isotope GEN CHEM Isotope Practice Worksheet Here are three isotopes
What does the number next to isotopes signify? Which isotope of lead is. Complete the following table, using the periodic table in the back of your book. The number 6 refers to the _____ c. For each of the following isotopes, write the # of protons, neutrons, and electrons.
Isotope Practice Worksheet —
Which isotope of lead is. For each of the following isotopes, write the # of protons, neutrons, and electrons. Here are three isotopes of an element: Students will practice how to write isotopes and how to calculate the number of subatomic particles based on atomic mass. The number 6 refers to the _____ c.
Isotope Practice Worksheet
Complete the following table, using the periodic table in the back of your book. What does the number next to isotopes signify? For each of the following isotopes, write the # of protons, neutrons, and electrons. Fill in the isotope names and any missing information, including. The number 6 refers to the _____ c.
Isotope and Ions Practice Worksheet Part I Isotopes PDF Isotope
For each of the following isotopes, write the # of protons, neutrons, and electrons. Here are three isotopes of an element: Complete the following table, using the periodic table in the back of your book. The average atomic mass of a lead atom is 207.2 amu. Students will practice how to write isotopes and how to calculate the number of.
Isotopes Worksheet Chemistry Isotopes Worksheet
Here are three isotopes of an element: The average atomic mass of a lead atom is 207.2 amu. Complete the following table, using the periodic table in the back of your book. Which isotope of lead is. What does the number next to isotopes signify?
Chemistry Worksheets Isotope Notation
Fill in the isotope names and any missing information, including. Complete the following table, using the periodic table in the back of your book. The average atomic mass of a lead atom is 207.2 amu. Here are three isotopes of an element: Which isotope of lead is.
Students Will Practice How To Write Isotopes And How To Calculate The Number Of Subatomic Particles Based On Atomic Mass.
What does the number next to isotopes signify? The average atomic mass of a lead atom is 207.2 amu. Fill in the isotope names and any missing information, including. For each of the following isotopes, write the # of protons, neutrons, and electrons.
The Number 6 Refers To The _____ C.
Complete the following table, using the periodic table in the back of your book. Here are three isotopes of an element: Which isotope of lead is.